Abstract
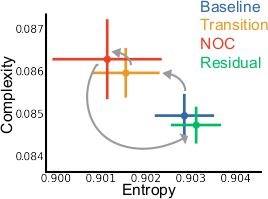
Non-ordinary states of consciousness (NOC) offer a way to examine how large-scale brain dynamics reorganize as experience changes. We studied a participant able to reliably enter a self-induced NOC state characterized by vivid imagery, altered bodily perception, and a sense of unity. Across 20 fMRI sessions, we measured functional connectivity in four conditions (Baseline, Transition, NOC, and Residual) and compared the results with a matched control group. During the Transition phase, connectivity became more variable, indicating a temporary destabilization of network organization. In the NOC state, inter-network connectivity decreased broadly, with visual cortex showing reduced coupling to auditory, sensorimotor, orbitofrontal, thalamic, and cerebellar regions, and the somatomotor-dorsal network disengaging from auditory and language cortices, paralleling the reported visual phenomena and changes in bodily experience. In contrast, frontoparietal and salience networks showed increased coupling with precuneus/posterior cingulate, multimodal temporal cortex, and cerebellar hubs, in agreement with subjective reports of sustained inward-directed attention and stable absorption. Entropy and complexity analyses revealed systematic shifts that tracked the experiential sequence and returned to baseline in the Residual condition. This single-case study brings together something uncommon: controlled experimentation, voluntary induction of NOC states, and rich phenomenological data. Taken together, these elements offer a strong foundation for neurophenomenological research and illustrate why pairing structured paradigms with lived experience is useful for understanding non-ordinary states of consciousness.